The functional fear of water
October 14, 2025
Introduction
In cold and wet conditions, water is the most effective driver of heat loss. When clothing and equipment absorb moisture, thermal conductivity increases, and the body’s own thermoregulation loses efficiency. When water governs heat loss, protection is no longer about comfort – it is about control, and in many cases, survival.
UR’s work with hydrophobic materials builds on the same physical and physiological principles that underpin the Banak Model – developed through collaboration between the Norwegian Armed Forces and the Norwegian Air Ambulance as a shared framework for understanding and managing hypothermia in the field. The model describes how water, wind, and inactivity together accelerate heat loss and reduce the body’s ability to maintain core temperature. It provides a clear basis for understanding why barrier and water protection are decisive in operational environments.

Moisture as the Main Driver of Heat Loss
When clothing or equipment becomes wet, the body’s heat balance changes dramatically. Water fills the air gaps that typically insulate and alters how heat moves between the body and the environment. Three processes co-occur:
Conduction – water has roughly 25 times higher thermal conductivity than air. Once textiles are saturated, direct heat transfer from skin to the environment increases sharply.
Evaporative cooling – each gram of evaporated water removes about 2.4 kJ of energy from the body. Even small amounts of moisture can therefore cause substantial heat loss.
Collapse of insulation – wet textiles can lose up to 60% of their insulation capacity because the air layers that trap warmth are replaced by water. This is a weakness of all moisture-absorbing fabrics, especially synthetic materials such as polyester, nylon, and microporous membranes.
Once garments are wet, the body must expend much more energy to maintain core temperature – energy that is diverted to drying fabric rather than preserving warmth. This is why water, not cold alone, is the primary driver of hypothermia risk.
Hydrophobia – Inherent Resistance to Water
The word hydrophobic comes from Greek – hydro meaning water, and phobos meaning fear. In practice, it describes a material that simply does not allow water to adhere. This resistance forms the foundation of true water protection.
Where water is the primary driver of heat loss, hydrophobic materials are the most effective countermeasure. Such materials have low surface energy and lack polarity – meaning water molecules cannot bond with the surface. Instead, droplets collect and roll off.

Nature as Engineer – The Lotus Effect
In nature, hydrophobic surfaces are not rare – they are perfected. The lotus leaf stays clean and dry even in tropical rain because its microstructure traps a thin layer of air beneath each droplet. Water never touches the plant’s surface, which means no heat is lost through conduction or evaporation. The lotus flower even maintains warmth in cool air – not only because it generates heat metabolically, like we humans do, but because its hydrophobic surface prevents that heat from being lost.
Hydrophobicity can be achieved either through chemical treatments or by the intrinsic molecular structure of the polymer itself. Bio-Based Dyneema® (ultra-high-molecular-weight polyethylene, UHMWPE) belongs to the latter category – it is hydrophobic by nature. The material consists of long carbon-hydrogen chains that are highly oriented and crystallized under tension, resulting in water absorption below 0.01%, a contact angle around 100°, and no microscopic pores or capillaries. (The bio-based version is chemically identical to conventional Dyneema® but derived from renewable feedstocks to reduce carbon footprint.)
Unlike fabrics that rely on surface coatings (DWR, fluorocarbon, silicone) for water repellency, Dyneema® remains hydrophobic regardless of wear, UV exposure, or cold. The property is not added to compensate for a weakness – it is part of the material’s nature. This makes it stable, predictable, and maintenance-free. As external conditions change, its characteristics remain constant. That fundamental stability distinguishes a hydrophobic material from a “water-repellent” textile: the latter gradually loses performance, while the former maintains its protection under any load.
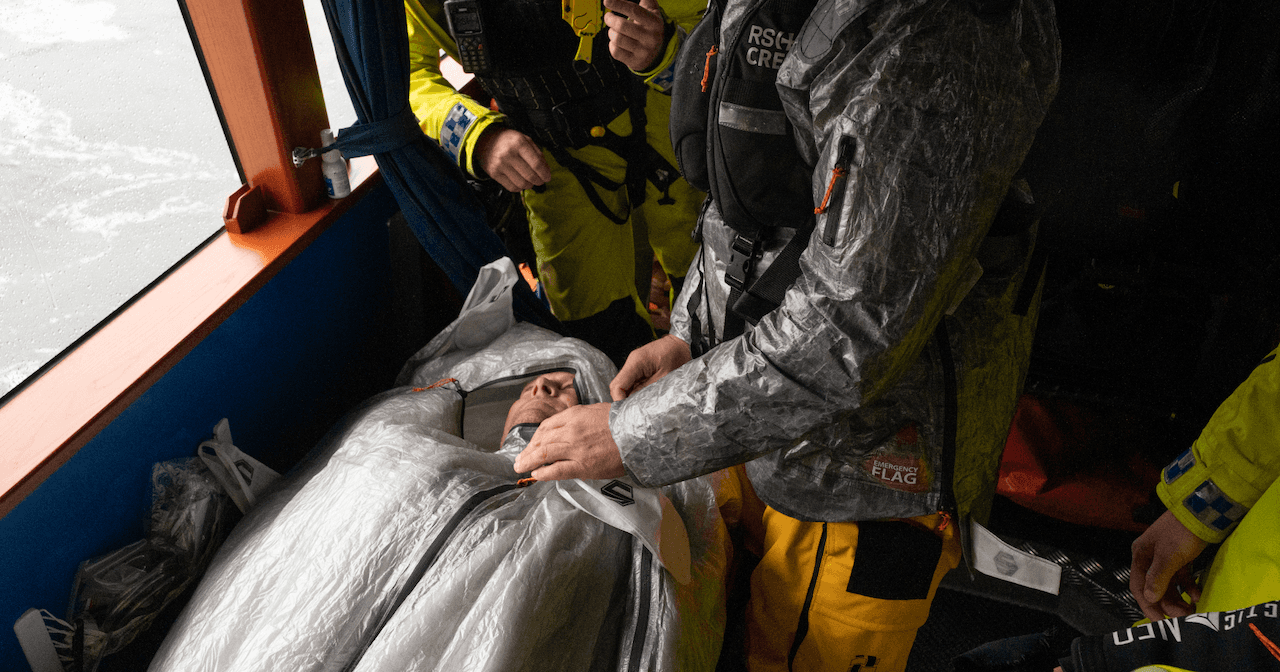
In practical use, UR’s Rescue Bag and ShelterWear maintain the same degree of protection over time – not because the material is treated, but because it remains unchanged.
A material that does not change provides the body with a stable, predictable environment for thermoregulation. This marks the transition from physics to physiology – from the material’s role to the body’s own response.
Active and Passive – Two Phases, Two Principles
The skin is the only truly intelligent shell we have. It regulates heat and moisture with precision, while membranes and so-called “breathable” fabrics are often described as smart – without being so. Working together with the body’s thermal control center, the hypothalamus, the skin maintains core temperature regardless of climate or activity level.
The body’s core temperature normally remains around 37 °C, while skin temperature in thermal equilibrium lies between 32 °C and 34 °C. When skin temperature drops below roughly 33 °C, sweating is automatically reduced – the body’s need for evaporation ceases. This marks the transition from the active phase, in which heat is produced and released, to the passive phase, in which heat is conserved.
Evaporation from the body occurs in two distinct ways — sensible and insensible perspiration.
The first is the visible one: sweating, the body’s deliberate mechanism for cooling through evaporation.
The second is constant and invisible — tiny amounts of water vapor escape continuously through the skin and airways, even when we are not sweating.
During intense activity and heat, sensible perspiration dominates. In cold and resting conditions, however, it is insensible evaporation that quietly drains body heat — often unnoticed. This means that even in a seemingly dry sleeping bag, we continue to lose warmth through invisible vapor transfer.
A truly hydrophobic system, such as Dyneema®, interrupts this process by preventing vapor from migrating away from the body in the first place. By maintaining a stable, saturated microclimate close to the skin, it eliminates evaporative heat loss and allows the body to regulate temperature efficiently.
This principle explains why vapor barrier liners (VBLs) are used in extreme cold. By halting moisture transport, they stabilize heat balance, reduce energy expenditure, and prevent cooling when activity ceases. The same principle underlies UR’s systems – the material should not compete with the body, but provide it with a neutral environment in which to function.For a deeper discussion of the “breathable and waterproof” paradox, see You Cannot Have Both.
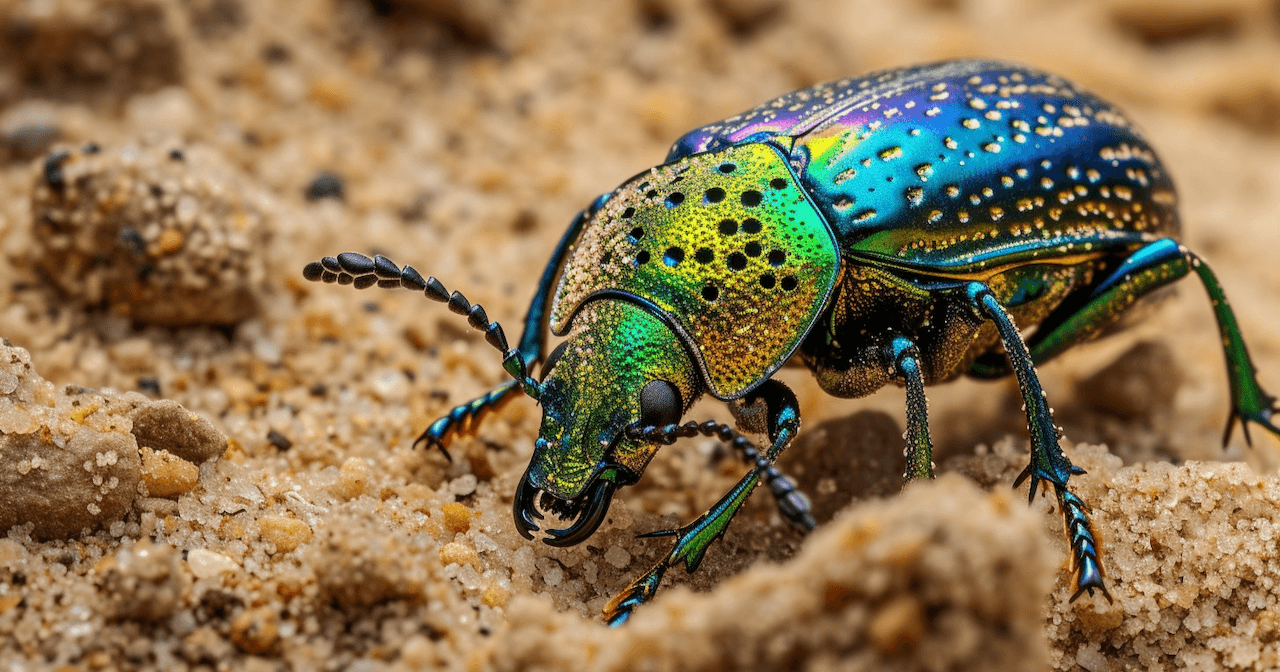
Nature as Engineer - The Intelligent Shell
The Namib Desert Beetle uses a pattern of hydrophilic and hydrophobic surfaces to collect moisture from desert fog. Its shell, too, is intelligent – designed not to reject water, but to direct it.
Defence, Research and Operational Relevance – The Principle of Durable Water Protection
The need for durable protection against water and moisture is not theoretical but an operationally documented requirement. It is clearly defined in NATO’s Defence Innovation Accelerator for the North Atlantic (DIANA) under Operations in Extreme Environments:
“Hydrophobic textiles, bio- and advanced materials for clothing, equipment or smart uniforms designed to protect against water ingress, hypothermia and heat shock.”
The statement points to more than hydrophobicity alone – it describes the need for materials and systems that remain waterproof over time, independent of chemical treatments or porous membranes. This is the same challenge identified in the Banak Model: once water gains access, the body quickly loses control of its thermal balance.
UR’s work therefore extends beyond making materials hydrophobic – it focuses on developing integrated systems that combine barrier performance, stability, and predictability.
Durable waterproof performance plays distinct yet critical roles across UR’s systems.
In our Rescue Bags, Dyneema® serves as both a vapor barrier and an external shell. The inner, sealed surface prevents evaporation and preserves body heat in accordance with the Banak Model, while the outer layer protects against rain, snow, and wind. Together, they create a stable, enclosed microclimate where the body does not have to expend energy drying fabrics or fighting moisture. The smooth, hydrophobic structure also provides a hygienic advantage – it repels fluids, prevents bacterial buildup, and can be easily cleaned in the field.
In our ShelterWear, the same principle is directed outward. Here, the dense outer shell forms an effective shield against the elements. An insulated version includes a ventilating liner and insulation that delays moisture rebound toward the body throughout the night. It’s liberating to be outdoors in rough weather with such a shell – you know you’ll stay dry, and you know you won’t be bringing damp gear into the tent. You shake it clean and dry, leave it outside to protect your equipment, or use it as a groundsheet while resting.

UR as Engineer - The Functional Barrier
Made from bio-based Dyneema®, Lun 1.0 embodies the physics of true protection: a shell that neither absorbs nor breathes, but remains constant.
Conclusion
The work on waterproof and hydrophobic systems is ongoing. We look forward to continued testing in both field and laboratory settings and to sharing insights with research and rescue communities working in cold, wet and pre-hospital environments. The goal is shared: to develop better equipment that enables people to protect themselves, their teams – and, for those in rescue and pre-hospital care – to save lives under demanding conditions.




